pyCapsid Report
October 16, 2023
Input structure
Identifier: 2xyy
Number of residues in the asymmetric unit: 2912
Number of protein chains in the asymmetric unit: 7
Multiplying factor to generate the complete protein complex: 60
-
If the multiplying factor is one (m = 1), the protein complex and the asymmetric unit are the same.
-
If the multiplying factor is larger than one (m > 1), the protein complex is m times the asymmetric unit.
Number of residues in the protein complex: 174720
Number of protein chains in the protein complex: 420
Figure: Asymmetric unit (below). Ribbon diagram of the protein complex’s asymmetric unit.
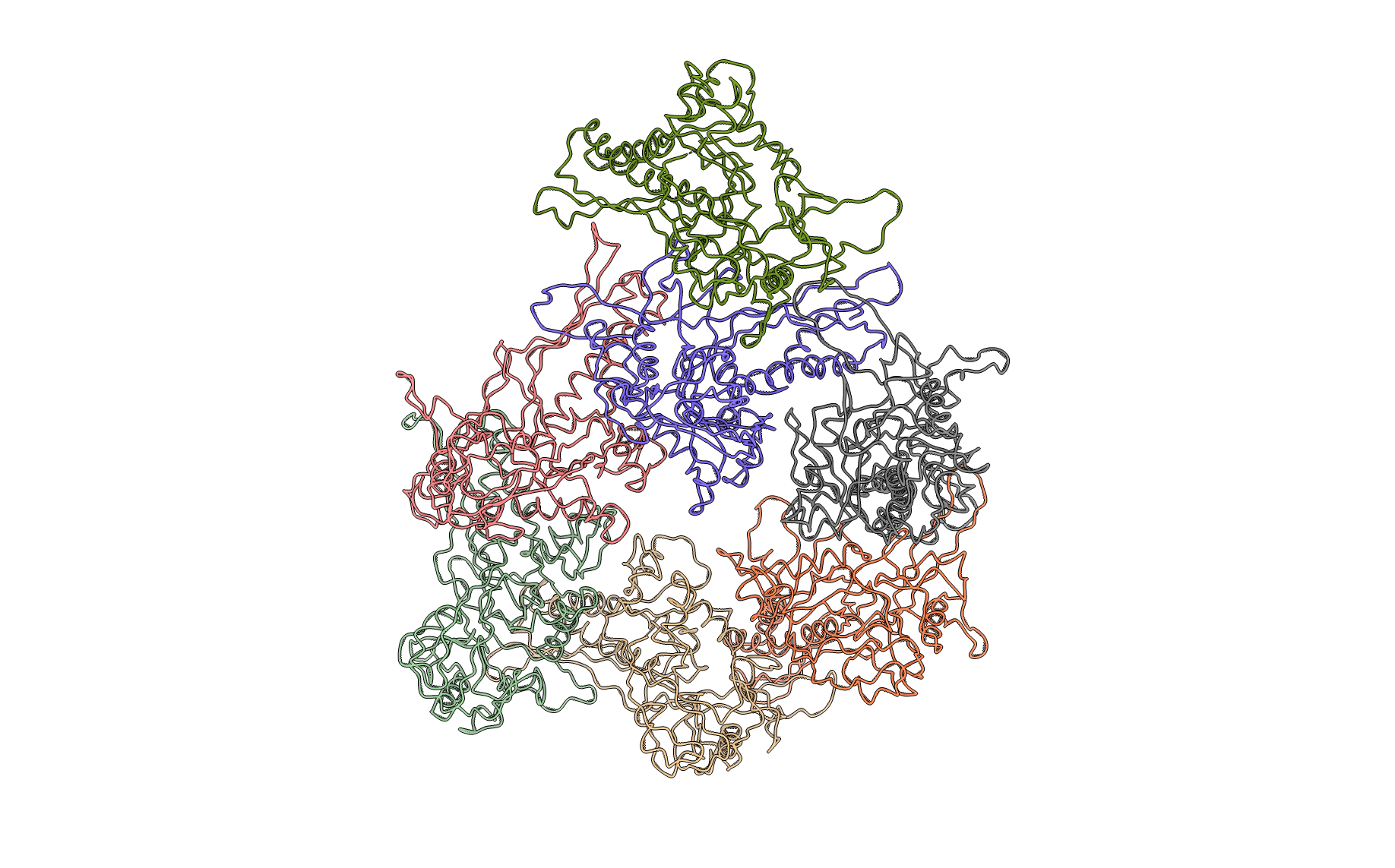
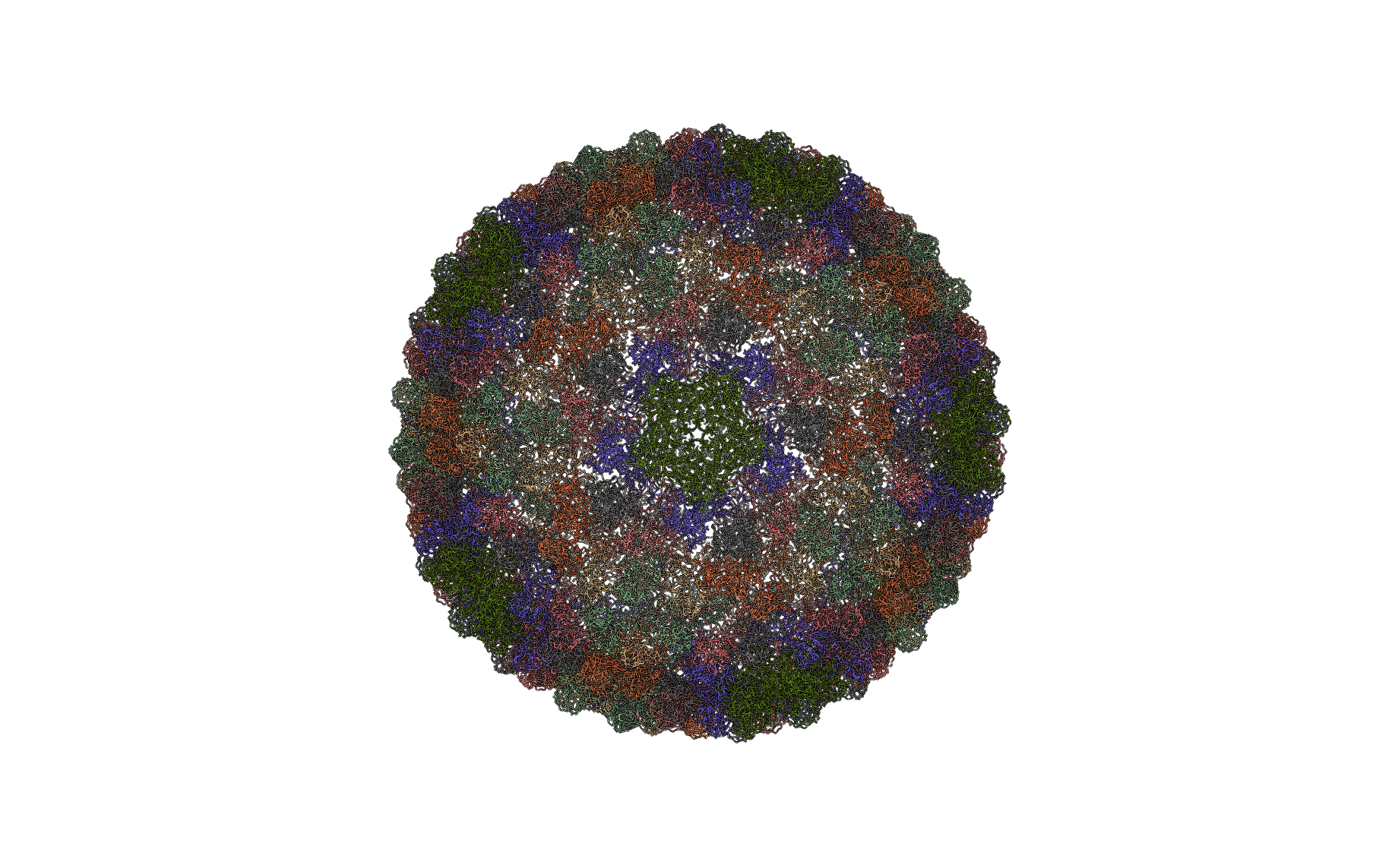
Figure: Full protein complex (below). Ribbon diagram of the full protein complex.
Elastic network model
Elastic model used: U-ENM
Calibrated stiffness constant (gamma): N/A
- The molecular model had 0 b-factors, and for this reason the results for the spring constant, correlation coefficient, and # of modes used aren’t provided.
Figure: Model calibration (below). The empirical B-factors are plotted against the predicted fluctuations. A regression line was fitted. From the slope (a), the calibration constant (gamma) was obtained using the formula: gamma = (8(pi)^2)/(3a).
Normal mode analysis (NMA)
Optimal number of modes reproducing B-factors: 4000
Correlation between empirical and predicted B-factors: 0.0
Figure: Correlation of B-factors (below). Empirical (blue) and predicted (orange) B-factors for each residue in the asymmetric unit.
Quasi-rigid mechanical units
Number of optimal quasi-rigid mechanical units identified: 420
Figure: Optimal clustering selection (below). Quality score (top) and number of unique clusters (bottom) obtained as a function of the number of clusters tested. The black lines in each plot higlight the quality score and number of unique clusters for the optimal number of clusters identified.
Figure: Quasi-rigid clusters (below). Ribbon representation of the complete structure, with each residue colored according to its cluster membership. Residues with the same color are members of the same quasi-rigid cluster.
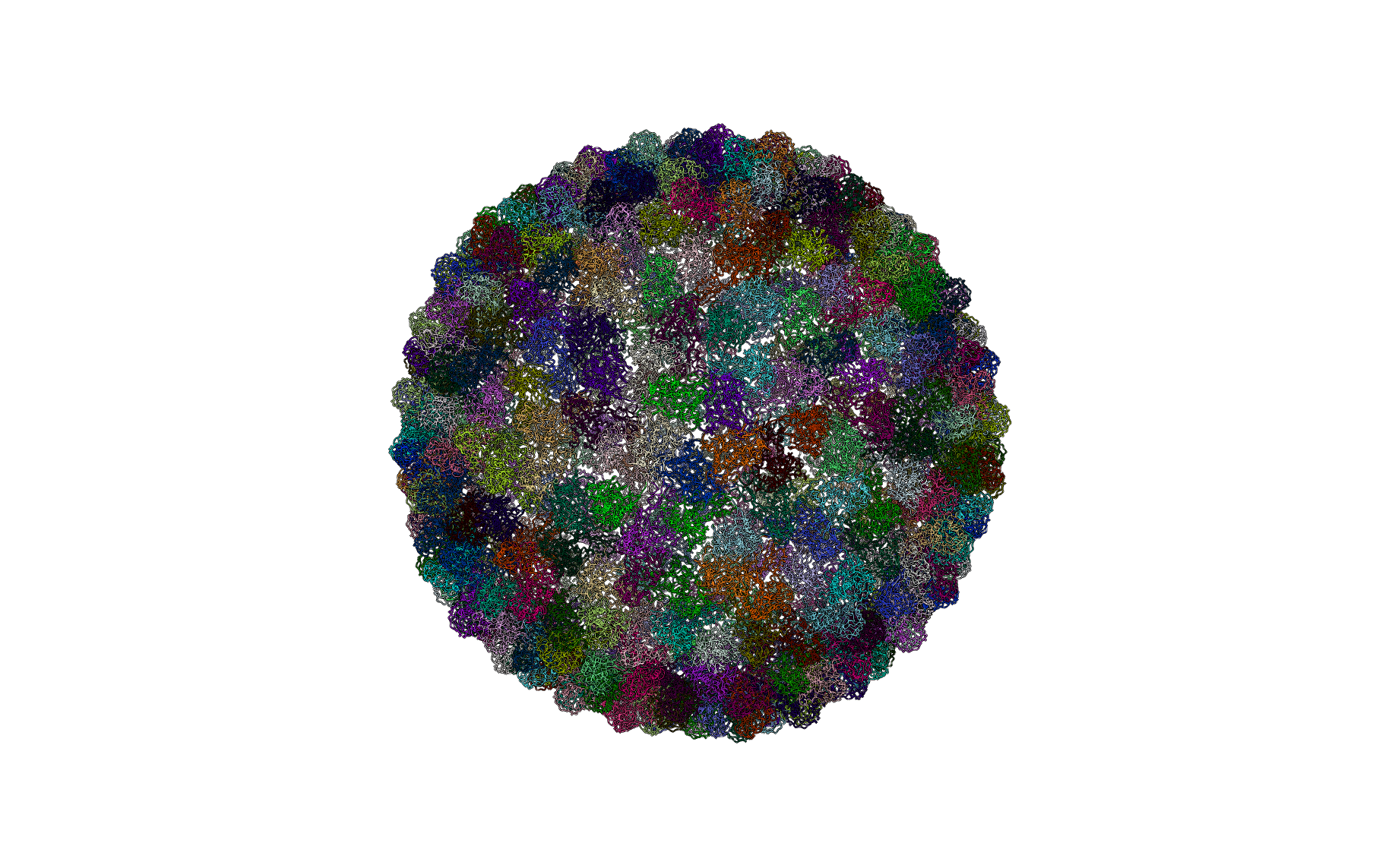
Figure: Residue cluster quality score (below). Ribbon representation of the complete structure, with each residue colored according to its cluster quality score. This is a measure of how rigid each residue is with respect to its cluster. Blue residues make up the cores of rigid clusters, and red residues represent borders between clusters.

Missing images in the report?
To render the missing images locally using ChimeraX, check the ‘chimerax’ folder of the report, and follow the instructions provided in the readme file. This will automatically place the images in the .md and .html versions. For the word document, copy and paste the relevant images directly into the document.
If you were visualizing the results using NGLView, you should have several images downloaded corresponding to the relevant images. After extracting “pyCapsid_report.zip”, place the corresponding images from your download folder into “pyCapsid_report/figures/structures/” replacing the empty images in that folder. This will fix the issue for the .md and .html versions. For the word document, copy and paste the relevant images directly into the document.